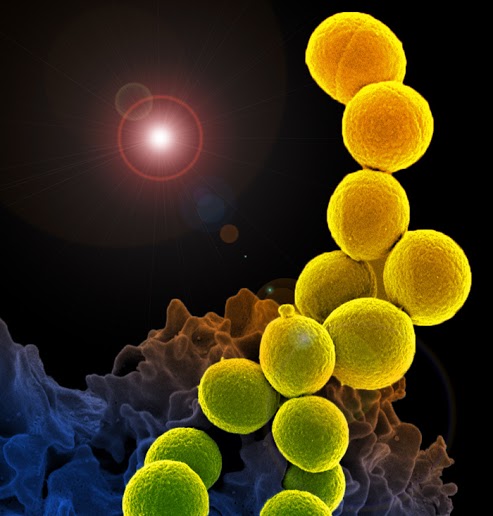
Stop Pill: one course of antibiotics can mess with your gut for 12 months
If self-medication is an Indian disease, antibiotics are its holy grail.
This prescription-only form of medication - used fairly conservatively in most parts of the world - is something we pop fairly unthinkingly here. We buy them over-the-counter to treat a toothache. Our first port of call to tackle a stomach or throat infection is a round of antibiotics. And even doctors treat them as a fairly routine treatment for a revolving roster of diseases.
There has always been a serious fallout of that: antibiotics resistance. But now new research tells us that something else happens post even a single course of antibiotics: your gut microbiome gets messed up for up to 12 months!
What is gut microbiome?
It's the population of microbes in our intestines.
The bacteria in our gut play a role in digestion (hence the great nutritional push for probiotics). When the stomach and small intestine are unable to digest certain foods, gut microbes help.
They also aid the production Vitamins B and K and help build immunity.
In other words, good bacteria in your gut keep your digestion and health running smoothly.
How antibiotics work
When we pop an antibiotic to fight an infection, antibiotics kill the bad germs - but they also kill the good germs.
The collateral damage is unavoidable - and so, too, are antibiotics some of the time. But if antibiotics become first port of call, research shows we're messing with our body's basic digestive processes for 12 up to a year each time.
What the new research says
A new study published in medical journal mBio suggests that antibiotics may have more side effects than previously thought - at least in the gut.
Researchers conducted two randomised, placebo-controlled trials of healthy people.
The joint trials, led by Egija Zaura at the University of Amsterdam, followed 66 healthy participants - 29 in Sweden and 37 in the United Kingdom.
The two antibiotics given in the Swedish trial were a lincosamide (clindamycin) and a quinolone (ciprofloxacin). The UK trial included a tetracycline (minocycline) and a penicillin (amoxicillin).
ALSO READ: Just how bad - or good - is chocolate for you really? We sorted through the data to answer that
"A single course of oral antibiotics altered the composition and diversity of the gut microbiome for months, and in some cases up to a year," the researchers reported.
Gut microbial diversity was significantly altered by all four kinds of antibiotics, which lasted for months.
In participants who took ciprofloxacin, microbial diversity was altered for up to 12 months.
Researchers also observed that some antibiotic treatments caused a spike in genes associated with antibiotic resistance, which is a whole other type of fallout.
Good germs, bad germs
Researchers found that clindamycin killed off microbes that produce butyrate, a vital short-chain fatty acid in your gut that helps control inflammation, carcinogenesis, and oxidative stress.
Those changes can also alter microbiome activities, including those that affect the immune system and digestion.
"My message would be that antibiotics should only be used when really, really necessary," Zaura said. "Even a single antibiotic treatment in healthy individuals contributes to the risk of resistance development and leads to long-lasting detrimental shifts in the gut microbiome."
Also relevant to us in antibiotic-popping India: researchers found that UK participants' bodies had more antibiotics resistance than the Swedes. In Sweden, antibiotic usage has dropped sharply in the past two decades over concerns about resistance.
Antibiotics resistance is widely recognised as one of the most challenging problems in modern public health around the world; it occurs "when an antibiotic has lost its ability to effectively control or kill bacterial growth; in other words, the bacteria are "resistant" and continue to multiply in the presence of therapeutic levels of an antibiotic," according to Tufts.edu.






![BJP's Kapil Mishra recreates Shankar Mahadevan’s ‘Breathless’ song to highlight Delhi pollution [WATCH] BJP's Kapil Mishra recreates Shankar Mahadevan’s ‘Breathless’ song to highlight Delhi pollution [WATCH]](http://images.catchnews.com/upload/2022/11/03/kapil-mishra_240884_300x172.png)

![Anupam Kher shares pictures of his toned body on 67th birthday [MUST SEE] Anupam Kher shares pictures of his toned body on 67th birthday [MUST SEE]](http://images.catchnews.com/upload/2022/03/07/Anupam_kher_231145_300x172.jpg)